Environmental Effects of Air Pollution
By Rayna Higuchi
Introduction
Air pollution in the Earth’s atmosphere has increased greatly over the past several centuries, but, along with it, so has our understanding of its consequences.1 It has negative effects on both health and the biosphere, leading to the realization that this problem must be quickly addressed. Typically, human health is the issue at the forefront of people’s minds, because the damage is much more immediate. However, the environmental impacts of air pollution can be catastrophic as well. One of the greatest problems with changes in the environment is that the ramifications may not be felt until many years later; due to this long delay, an issue may not be addressed until it has already become a widespread problem. The main environmental concerns that have been identified in conjunction with air pollution are a reduction of stratospheric ozone, ocean acidification, the greenhouse gas effect, and acid rain.This article will address the issues arising from these phenomenons, as well as potential preventative and remedial solutions.
Ozone
The loss of stratospheric ozone is a major problem in certain parts of the world. It occurs in many ways, one of which is when chemicals released into the atmosphere react with the ozone and convert the O3 molecule into other forms. If enough ozone is lost, as in Figure 1, large amounts of UV radiation reach Earth’s surface. This causes an increase in rates of skin cancer in the human population seen in these affected areas, and interferes with photosynthesis by damaging the leaves of plants.2 One issue that has since been addressed was the release of chlorofluorocarbons, also known as CFCs.3 These were very damaging to the ozone layer and they were banned in 1996, resulting in a reduction in the concentration of these harmful molecules seen in the stratosphere.3 Completely stopping production of CFCs proved a very effective policy, though there are still other similarly reactive chemicals that are equally damaging. The best way to limit the release of these chemicals is to stop using them. A recent demonstration of countries working together to limit the release of a specific pollutant was displayed this past year, when over 170 countries gathered in Kigali to form an agreement about their release of hydrofluorocarbons (HFCs). HFCs are a major greenhouse gas, released by many nations worldwide. These Kigali Accords included both developing and developed countries, and signaled a change in the way environmental changes could be tackled, as it set specific goals on this pollutant that each nation could be held to.4 This method has yet to be tested, but the potential improvements are vast. It differs from past conferences in that the goals it sets are more concrete, and it focuses only on a singular key chemical. In essence, its main benefits are that it can hold countries accountable, thus providing a real standard for them to reach for.
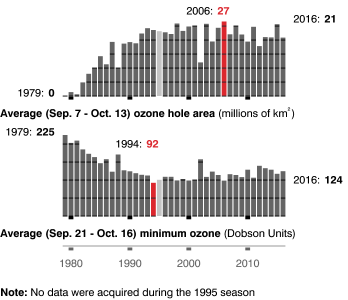
Figure 1. Bar graphs that showing the size of the ozone hole over Antarctica, where the ozone depletion is the greatest due to increase in reaction speed because of lower temperatures. The size of the “hole” has increased since 1980, and the minimum levels of ozone have decreased each year over that same period. Source: NASA.
Contrasting to this idea of stratospheric ozone loss is the increase in tropospheric ozone, which forms at ground level due to reactions between Nitrogen Oxides (NOx) and Volatile Organic Compounds (VOCs).5 Figure 2 shows the proper placement of the ozone layer, in the stratosphere. Below that is the air humans breathe, the troposphere. Ozone that forms here is called “ground-level”; there are numerous health effects of ground- level ozone, which can be found in the article on health effects due to air pollution. The primary environmental issue with ground-level ozone is that it harms plant production, resulting in millions of dollars in losses from agriculture.5 The best way to reduce this effect is to reduce the number of VOCs and NOx emissions, again through methods such as improving efficiency of industrial processes and transportation.
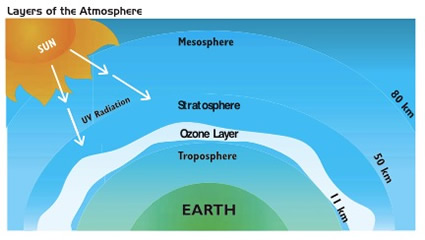
Figure 2. Image demonstrating the height of the ozone layer. Sunlight passes through the mesosphere and is stopped in the stratosphere at the ozone layer. When ozone is formed in the lowest level of the atmosphere, in the troposphere, it can cause irritation to humans and wildlife. Source: Association for Canadian Educational Resources (ACER)
Greenhouse Gases
Greenhouse gases include gases such as methane and carbon dioxide, as wells as the HFCs. They are not particularly reactive, nor are they harmful to human health in small amounts. However, their large-scale release in the past century has caused a global rise in temperatures, demonstrated in Figure 3. These are released from sectors such as agriculture, transportation, industry, and electricity generation.6 This temperature change phenomenon, known as global warming, has a plethora of negative effects, including the changing of weather patterns, the destruction of ecosystems, loss of land due to rising ocean levels, and the extinction of species.7
A global rise in ocean levels, demonstrated in the graph in Figure 4, harms both humans and the overall biosphere. It results in the loss of coastal lands, where many human populations live alongside coastal ecosystems. Whole communities, both human and otherwise, are destroyed by a change of just a few inches.
There are many solutions to this, including implementing new technologies, reducing the use of the technologies we already have that emit large amounts of greenhouse gases, and the transition to an electric grid that doesn’t run off of fossil fuels. This is most effective for areas in which renewable energy is actually a viable and cost- effective option, like implementing solar power in California or ocean turbines on coastal regions. Certain renewable energies are not practical in all locations, due to environmental factors– there is no point, for instance, in investing in solar panels in an area that does not get sufficient sun. The biggest issue with renewable energy is the lack of dependability, which is why new technologies need to be sought in order to make them as consistent as fossil fuels before they will ever be fully adopted. They also tend to be more expensive, which is why it is harder for developing countries to invest in this type of power. Thus, it falls on richer, more developed countries to participate in fossil fuel divestment, in which they gradually transition to an infrastructure that favors cleaner energies, and eventually end their participation in the fossil fuel market.
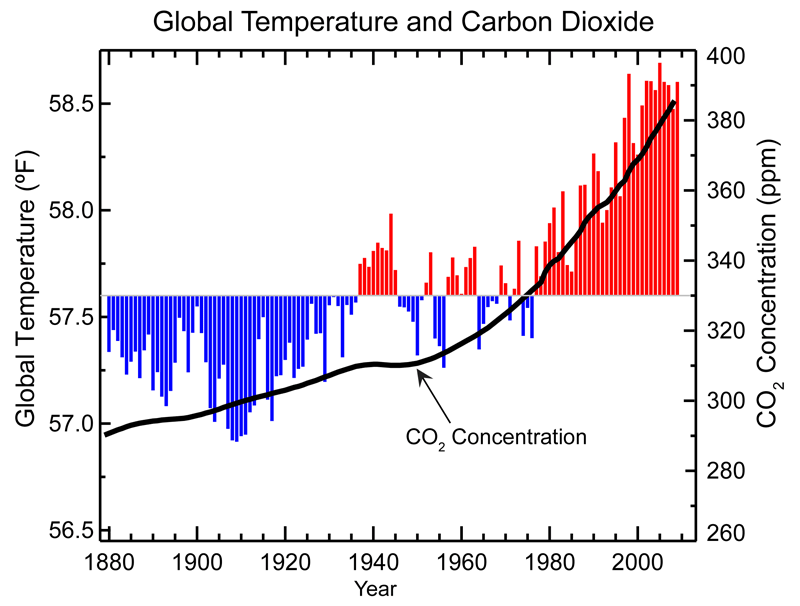
Figure 3: Graph relating the change in average global temperature over time, overlayed on a graph showing the concentration of Carbon Dioxide in the atmosphere. The trend shows the correlation between increase in the global temperatures and increase in carbon dioxide concentration. Source: National Climatic Data Center
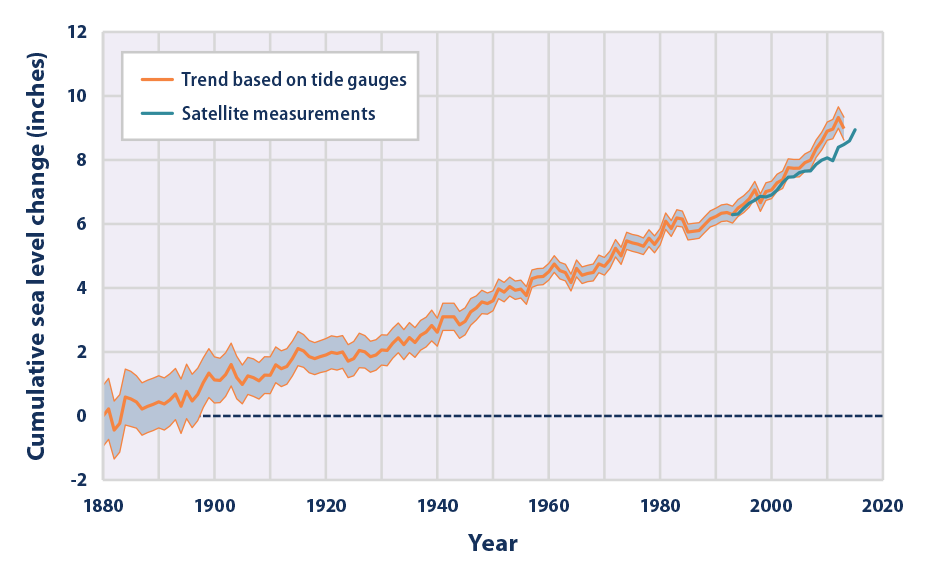
Figure 4. Graph indicating the change in average sea level since the year 1880. Source: US Environmental Protection Agency.
Ocean Acidification
The large amounts of carbon dioxide that have been released into the atmosphere have another effect. The ocean typically absorbs a large amount of CO2 from the air, almost 25% of the carbon released in any given year.8 Since the industrial revolution, the amount of carbon in the oceans has been increasing steadily. This was initially viewed as a good thing, as scientists thought the ocean could offset some of the carbon release in the atmosphere. However, decades of water research have demonstrated a very different situation unfolding: ocean acidification. The carbon dioxide that is being absorbed is fundamentally changing the chemistry of the ocean, and is actually shifting the pH of the waters downward, into a more acidic range.8 Figure 5, taken from the National Oceanic and Atmospheric Administration (NOAA) website, demonstrates the drop in pH of the ocean next to the increase in adsorbed CO2. Besides the solutions outlined previously, another way to address this problem is carbon sequestration. This is a term used to describe the process of removing carbon from the atmosphere and locking it in another state, where it cannot be released back into the troposphere. Methods include storing it in biochar, planting trees on a large scale, or drilling deep holes and injecting carbon dioxide gas into them. Each of these methods has its costs and benefits. Trees, for instance, do not store the carbon for as long as the other two methods, but are cheaper, and can later be put to use as wood products. Gas injection can be more expensive, but it sequesters the carbon for a very long time. Biochar can be used to reintroduce carbon into carbon depleted soils, but is difficult to do properly and if done wrong may release large amounts of methane. These costs and benefits must be analyzed on an individual basis for each country, in order to find the best solution for each area’s needs.
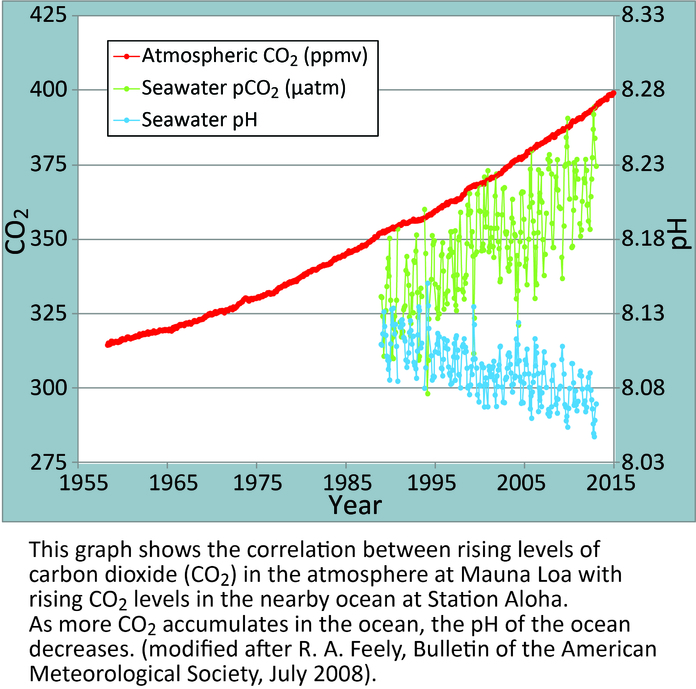
Figure 5. Graph demonstrating the correlation between Carbon Dioxide levels in the atmosphere and ocean and seawater pH. Source: National Oceanic and Atmospheric Administration (NOAA).
Acid Rain
Acid rain is also a major issue, and it is seen most in metropolitan areas. Sulfur dioxide and nitrogen oxides react with the water as if condenses and falls to the earth, turning it acidic.9 A picture demonstration of this process can be seen in Figure 6.
The pH level, which indicates how acidic or basic a solution is, varies greatly depending on the levels of the chemicals in the air, but any pH, no matter how extreme, can be damaging. Acid rain irritates skin, damages plants and reduces their ability to photosynthesize, and can even destroy cars and cement. One possible solution is to reduce the burning of coal, which releases enormous amounts of nitrogen oxides.9 Again, this is not necessarily practical everywhere, and new solutions to cleaner-burning materials, or other sources of energy, need to be found.
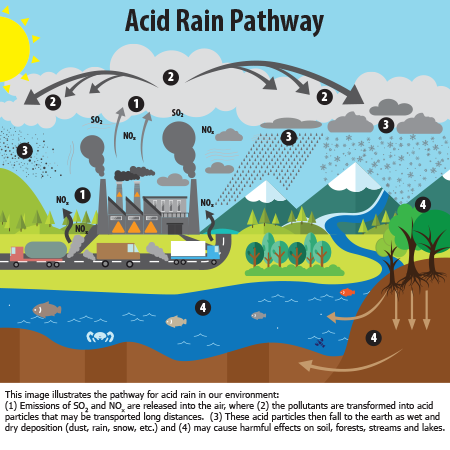
Figure 6. Image demonstrating the pathway of formation of acid rain. Source: US Environmental Protection Agency.
Conclusion
These are not the only environmental issues with air policy, but they are some of the most prevalent, and they demonstrate why it is imperative that the world transitions to a state of reduced net emissions of all harmful pollutants, and all greenhouse gases. There exist many other solutions (for more, visit the air pollution technology page) besides those outlined in this article, each of which provides an opportunity to reduce the chemicals that are so harmful to the environment. Individually, each of these solutions has very little impact. If implemented by all developed nations, however, which have the power and the resources to do so, they have the potential to remove many of the chemicals that are so damaging to the environment, and reduce their net greenhouse gas emissions. Developing nations, which often lack the stability or economics for any far-reaching reform, can still invest in many of these solutions. Renewable energies, for example, help limit their reliance on fossil fuels, a typically costly market. They also possess a unique feature that developed nations lack, which is the ability to form their infrastructure around these new technologies rather than limiting themselves to the old ones. Going forward, both types of nations will play key roles in the creation of a healthier Earth, making the choice to reduce air pollution critical.
